Hansen Solubility Sphere
Hansen solubility parameters were developed by Charles M.
Hansen in 1967 to predict the solubility of polymers in solvents. They are widely used in the
paint and coatings industry. The method is based on the idea that like dissolves like. This is the case when the solvent and the solute have similar Hansen Solubility Parameters.
The three Hansen parameters can be considered the coordinates of a point in the so-called Hansen space. The closer two points are, the more likely the
compounds are to dissolve into each other. In the case of a polymer,
only solvents within a certain range will dissolve the polymer. This range is
usually an ellipsoid and only solvents within this space are likely to dissolve
the polymer in question.
Hansen Solubility Sphere
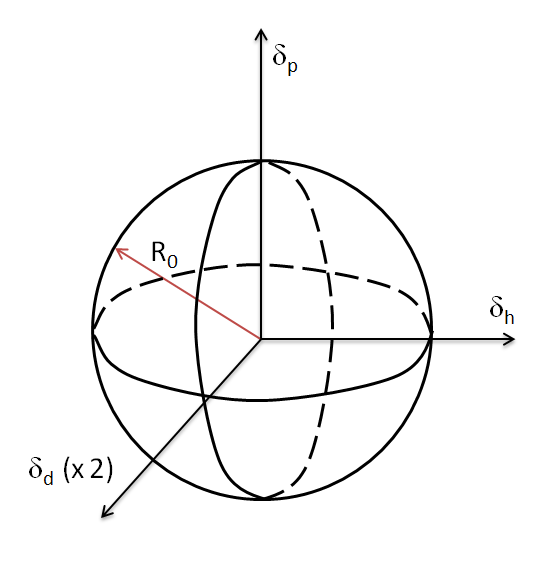
Occasionally, the scale of the dispersion axis is doubled, providing approximately a spherical volume of solubility. Then the distance of the solvent coordinates (δd2, δp2, δh2) from the center point (δd1, δp1, δh1) of the solute sphere is given by
Ra2 = 4(δd2 - δd1)2 + (δp2 - δp1)2 + (δh2 - δh1)2
The distance Ra in the equation above can be compared with the solubility radius of the polymer, R0. If Ra < R0 then there is a high likelihood of the solvent to dissolve the polymer. The radius of the solubility sphere is often called the interaction radius and the ratio Ra / R0 the relative energy difference (RED) of the system.
Ra / R0 > 1 → the compound is a non-solvent
Ra / R0 < 1 → the compound is a solvent
Ra / R0 = 0 → the compound may cause swelling
Hansen1 and Barton2 have tabulated the interaction radius and partial solubility parameters for many polymers and solvents.
To give an example, we calculate Ra / R0 of polystyrene (PS) and acetone. According to Hansen1, PS has an interaction radius of approximately 12.7 and its partial solubility parameters are (δd, δp, δh) = (21.3, 5.7, 4.3) and those of acetone are (15.5, 10.4, 7.0). This gives
Ra = {(2·15.5 - 2·22.3)2 + (10.4 - 5.8)2 + (7.0 - 4.3)2}1/2 = 12.8
→ Ra / R0 = 12.8 / 12.7 ≈ 1.0
This result indicates that acetone is not a solvent for polystyrene but might cause some swelling of the polymer.
| Compound | δp | δd | δh |
| Poly(vinylchloride), PVC | 8.8 | 18.6 | 5.8 |
| Polychloroprene, Neoprene | 4.3 | 19.5 | 3.1 |
| Polyethylene, PE | 0.0 | 17.6 | 0.0 |
| Poly(isobutylene) | 2.5 | 16.2 | 4.3 |
| Polypropylene | 0.0 | 18.0 | 0.0 |
| Nylon 6,6 | 5.1 | 18.2 | 13.7 |
| Poly(1,4-butadiene) | 2.3 | 17.3 | 2.6 |
| Polyisoprene | 1.1 | 16.9 | -0.4 |
| Poly(ethylene terephthalate), PET | 7.3 | 18.2 | 7.9 |
| Poly(ethyl methacrylate), PEMA | 7.8 | 17.9 | 3.4 |
| Poly(methacrylic acid) | 12.5 | 17.4 | 16.0 |
| Poly(methyl methacrylate), PMMA | 10.5 | 18.8 | 5.7 |
| Poly(acrylonitrile), PAN | 15.1 | 20.0 | 7.9 |
| Polystyrene, PS | 5.9 | 18.7 | 3.5 |
| Polysulfone | 8.8 | 18.7 | 6.1 |
| Poly(vinyl alcohol), PVOH | 12.5 | 17.5 | 10.0 |
| Poly(vinyl acetate) | 11.3 | 20.9 | 9.7 |
| Poly(vinyl butyrate), PVB | 4.4 | 18.6 | 13.0 |
| Poly(vinyl butyral) | 9.5 | 19.1 | 8.0 |
| Poly(tetrafluoroethylene), PTFE | 0.0 | 14.0 | 0.0 |
References
- Charles M. Hansen, Hansen Solubility Parameters: A User's Handbook, 2nd Edition, 2007
- Allan F.M. Barton, CRC Handbook of Solubility Parameters and Other Cohesion Parameters, 2nd ed., 1991